In this website an automated analysis of available data is made - the data should be treated in this regard and should not be utilised for any purpose beyond the exploratory viewing of equine influenza outbreaks. Please email us with any queries.
We define
outbreaks
as discrete foci of infection, usually a premises,
which may involve one or many animals.
We avoid using the term
case(s)
due to a lack of firm data on the actual number of animals affected.
Where multiple animals are reported to be clinically affected,
we often receive a sample from only one of the clinically affected animals for laboratory testing.
Please be aware that the outbreaks reported will only be reported if they have been
confirmed to be positive through laboratory testing of
one or more of the affected animals.
Therefore there may be outbreaks that are not reported as they have not undergone laboratory testing or have not been sampled at an optimal
time to obtain a positive test result.
The data presented must be interpreted with caution, as there is likely to be some bias in the way that samples are submitted for laboratory testing and subsequently reported. Consequently these data do not necessarily reflect true equine influenza frequency within the international equine population. A region with no reported outbreaks does not necessarily equate to the disease not being present in that region.
- This section depicts where and when equine influenza occurred in the UK and the rest of the world respectively for the period selected.
- Use the date inputs to select a date range of interest and press the ANALYSE button to run the analysis
- The maps will show where, at region level for the UK and country level for the world, reported outbreaks have occurred and coloured by number of outbreaks reported
- Outbreak curves will show the progression of outbreaks.
- This section contains links to reports, prior to 2019, on equine influenza occurrence in the UK
Select date range of interest
Run analysis
Click on an affected area for total outbreaks and info on reports from that area
Select date range of interest
Run analysis
Click on an affected area for total outbreaks
Veterinary Information
Introduction
Equine influenza is endemic in the UK and is a major cause of respiratory disease in horse populations around the world. It has been responsible for substantial socio-economic losses, with major outbreaks such as the one in Australia in 2007 costing the racing industry an estimated $1 billion AUD and halted racing in GB in 2019.
Current circulating viruses belong to the subtype H3N8 and, like human influenza viruses, undergo antigenic drift due to the gradual accumulation of mutations in the surface glycoproteins. In particular, changes in the viral haemagglutinin(HA) help the virus avoid immunity acquired from previous infection or vaccination. As a result, strains need to be closely monitored to ensure the effectiveness of the current commercially available vaccines.
Clinical signs
In unvaccinated horses the classic signs of equine influenza include:
- a harsh dry cough
- pyrexia
- nasal discharge (serous, mucoid or mucopurulent)
The infection will usually spread very rapidly through a naive horse population with close to 100% infection rate. Horses that have only partial protection, either due to irregular vaccination or the use of out-dated vaccine strains, will typically show signs of milder, non-specific respiratory disease. Vaccinated horses can also be infected and shed virus, but signs should be milder and they should shed less virus. Equine influenza should be considered as a differential in any vaccinated horses with non-specific respiratory signs.
Actions during an outbreak
1. Diagnosis
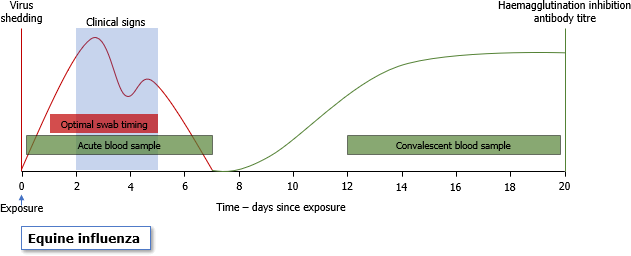
Obtaining a positive diagnosis on a premises is essential to implement successful control measures to reduce spread on and beyond a premises. It is also important to know if a horse has suffered from equine influenza as it will affect the length of time required for recovery post-infection, in order to prevent secondary complications. Nasopharyngeal swabs should be taken from those animals with the most recent history of coughing and ideally at least two to three animals should be sampled. If history suggests infection has been present on a yard for sometime and there are no recently coughing cases (within the last 7 days), to obtain a diagnosis of influenza, it would be advisable to take a serum sample from an unvaccinated animal affected >10 days previously.
2. Measures to control spread on and beyond the infected premises
If you suspect a horse has influenza, ideally infected cases should be isolated in a separate airspace, but given the contagious nature of the virus, spread on a premises may have already occurred prior to veterinary involvement. In such circumstances, implementing voluntary movement restrictions and alerting neighbouring premises (due to the potential for the virus to spread up to 1.5km in certain weather conditions), to contain the virus to the infected premises is advisable.
The virus can survive for limited periods outside of the host, so fomite transmission via clothing, tack and other inanimate objects such as brushes is also possible and must be considered. The virus is fairly labile so normal detergents are sufficient to kill it.
The virus can be spread easily and very quickly from horse to horse, with close to a 100% infection rate to be expected in a group of unvaccinated individuals. Most horses exposed to the virus will show signs within a period of 1 to 5 days.
3. Treatment of infected cases
Most cases will recover with rest and anti-inflammatories. It is advisable to feed cases from the ground and maintain a well-ventilated environment.
Damage to the epithelium and cilia of the upper respiratory tract caused by the virus leaves horses with increased susceptibility to secondary opportunistic infections. The development of secondary bacterial infections is a very important complication of equine influenza. Prolonged pyrexia, lethargy and malaise are usually accompanied by a more profuse mucopurulent/purulent nasal discharge and such cases may require treatment with anti-microbials.
Surveillance scheme
Monitoring virus changes
The HBLB equine influenza surveillance scheme was set up to monitor genetic and antigenic changes in equine influenza viruses circulating in GB. It provides free advice and free PCR testing of suspect cases for equine influenza, to all practices registered with the scheme. It also provides a means for rapid communication with veterinary practitioners in the face of an outbreak.
The information that is collected allows comparison of currently circulating viruses with those used in commercial vaccines. These data are used to determine whether current strain recommendations should be updated or not.
Vaccine strains
Official recommendations for vaccine strains are made annually by the World Organisation for Animal Health (OIE). For more information about current recommendations please go here.
Influenza in dogs
In some parts of the world such as the USA and China, equine influenza has also infected dogs. After its emergence in 2003 in the USA, the disease spread rapidly between states and has now adapted to transmit efficiently in the dog.
Cross-species transmission of the equine virus from horses to dogs has also been observed on occasion. In the UK there have been two confirmed outbreaks of H3N8 equine influenza in foxhounds, both retrospectively diagnosed. Equine influenza was also diagnosed in dogs in Australia following the large outbreak in horses in 2007. Most of those horses had no protection and could therefore shed large quantities of virus. Dogs kept in close contact with infected horses seroconverted to equine influenza.
Clinical signs in dogs include:
- Persistent harsh cough (despite treatment with antibiotics)
- Nasal discharge
- Fever
- Increased respiratory rate and effort
- Rapid spread within a group of dogs
These signs usually appear two to five days after exposure to the virus. Where there is the possibility of respiratory disease in dogs being due to equine influenza infection, such as after close contact between dogs and infected horses, diagnostic tests to detect virus in horses can be applied to respiratory samples taken from affected dogs. However, as canine influenza has not been diagnosed as an active transmissible disease in dogs in the UK, there is currently no vaccine available for it.
Additional sources for information
Vaccination Information
Current recommendations and vaccines commercially available in the UK
Like other influenza viruses, equine influenza constantly changes so vaccine strains need to be updated periodically. Equine influenza viruses isolated from all over the world are analysed to determine if the current strains in commercially available vaccines are adequate to provide an acceptable level of protection to these current circulating field strains. Epidemiological information obtained from outbreaks is also utilised to determine if vaccine strain updates may be required. As part of this process the Horserace Betting Levy Board (HBLB) sponsors surveillance of equine influenza in Great Britain. An independent panel of worldwide experts, including UK scientists, meet every year to select strains, as part of the World Health Organisation's (OIE) Expert Surveillance Panel (ESP).
The official recommendations for equine vaccines are approved by the OIE and are available here.
The current recommendations are that vaccines for the international market should contain both clade 1 and clade 2 viruses of the Florida sublineage:
Clade 1 is represented by A/eq/South Africa/04/2003-like or A/eq/Ohio/2003-like viruses. Clade 2 is represented by A/eq/Richmond/1/2007-like viruses. The recommendations have not been changed since 2010. Two vaccines available in the UK contain a recommended Clade 1 strain, only one product contains a recommended Clade 2 strain
| Vaccine name | Noah compendium datasheet | Vaccine type | Viral strains included | Conforming recommendations | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1995 | 2004 | 2010 | |||||||
| Equip F | Link | Whole inactivated Sub-unit vaccine adjuvanted with ISCOM |
|
✔ | ✗ | ✗ | |||
| Equilis Prequenza | Link | Whole inactivated Sub-unit vaccine adjuvanted with ISCOM |
|
✔ | ✗ | ||||
| ProteqFlu | Link | Canarypox viral vector expressing only EI virus haemagglutinin |
|
✔ | |||||
Vaccination regulations and dosing regimes
To ensure that horses gain the maximum protection from vaccination, vaccines need to be given according to the manufacturer's instructions on the product label.
Previously, the FEI and BHA regulations allowed vaccination within a wider range of dates than the datasheets, however the BHA regulations have been recently amended (Jan 2022) to align more with those stated in the vaccine datasheets. To ensure the optimum performance from any one type of vaccine the product label should be followed as accurately as possible.
 |
Previous interval | Interval from 1 JANUARY 2022 |
|---|---|---|
| V1 | Day zero | Day zero |
| V2 | 21 - 92 days | 21 - 60 days |
| V3 | 150 - 215 days | 120 - 180 days |
| Booster | Not more than 1 year apart | Not more than 6 calendar months apart |
Adverse events, reactions and their reporting
Any adverse event that a horse experiences after receiving an influenza vaccination should be reported to the vaccine manufacturer. This is one of the major ways the manufacturers monitor both the safety and efficacy of their products.
The Veterinary Medicines Directorate (VMD) also monitors the safety and efficacy of veterinary products in the UK.
Adverse events include
- Suspected failure to protect - i.e. a horse that develops signs of influenza when a product is used according to the label.
- Any reactions, clinical signs or illness that follows injection either on or off label
Adverse reactions should be reported directly to the vaccine manufacturers. If a horse has received more than one type of vaccine then it is the manufacturer of the last vaccine given that should be reported to.
Influenza in Vaccinated Horses
We would particularly like to hear from you if you have a case of influenza that has been confirmed by laboratory testing in a horse that has been vaccinated on-label. Email us to make contact.



